The Laboratory Reveals for the First Time that the new function of GFAT1can as a Signal Transduction Molecule in Cell Signaling Regulation: lung adenocarcinoma cells Rely on GFAT1...
2022-08-161401On August 9, 2022, the a joint research article was published on the journal Cell Discovery, with the title of “GFAT1-linked TAB1 glutamylation sustains p38 MAPK activation and promotes lung cancer cell survival under glucose starvation” by the SKLRD’s research groups led by Chen Tao and Liang Wenhua and the research group led by Jiang Yuhui of Renji Hospital affiliated to Shanghai Jiaotong University School of Medicine. This paper revealed a new mechanism of lung adenocarcinoma cells depending on the hexosamine pathway metabolizes enzymes (GFAT1 )to promote p38 activation and cell survival under glucose starvation; it was the first to report that GFAT1 protein can function as a signal transduction molecule in the cell signaling regulation.
Abnormal cellular metabolism is one of the important causes of tumorigenesis. Tumor cells undergo metabolic (pathway) reprogramming to provide their own unlimited proliferation with the required energy and biomacromolecules; in this process, abnormally expressed metabolic enzymes play a crucial role [1]. Besides, metabolic enzymes can also perform functions other than regulating metabolism, where the transduction of signaling pathways helps tumor cells cope with the survival pressure [2].
The hexosamine pathway is an important branch of glucose metabolism, playing an important role in maintaining cell homeostasis. The study finds that it is overactivated [3,4] in various tumor cells. Glutamine-fructose-6-phosphoaminotransferase (GFAT1), a key rate-limiting enzyme in the hexosamine pathway, is highly expressed in some tumor cells and is associated with poor prognosis in patients[5,6]. Although the regulation of cell survival by GFAT1 has been widely reported[7,8], it is unclear whether it can perform functions other than the regulation of (hexosamine) metabolism.
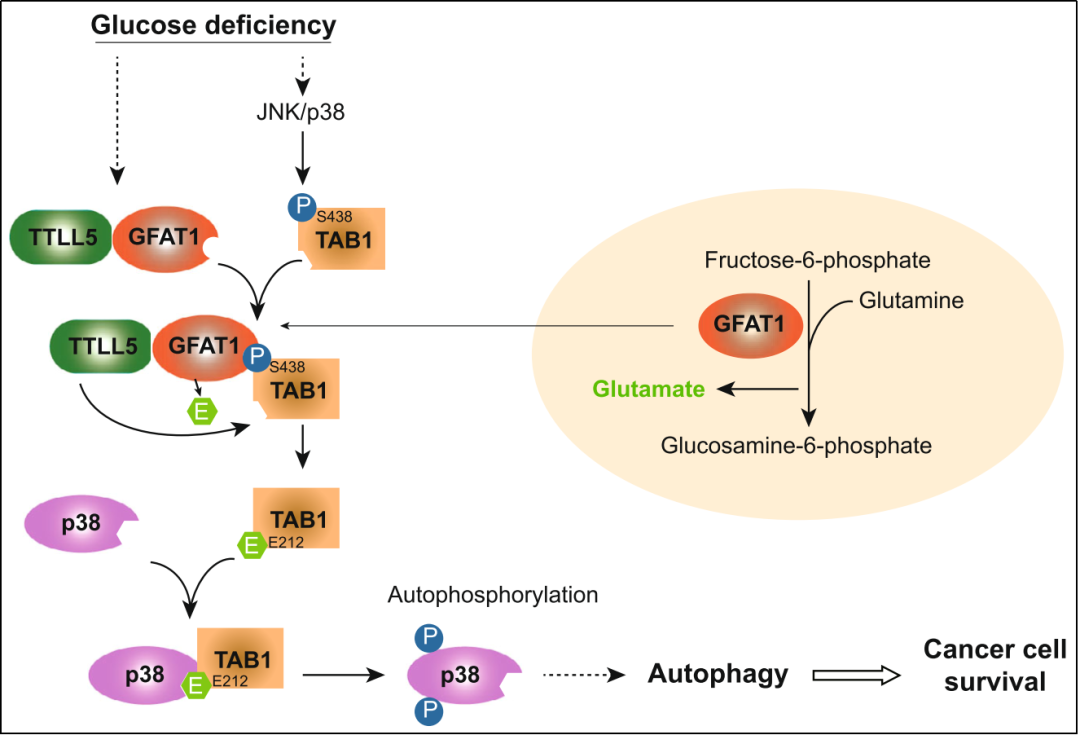
This study revealed that GFAT1 was abnormally expressed in lung adenocarcinoma cells. Upon knockout of GFAT1, the survival of lung adenocarcinoma was inhibited in glucose-deficient conditions, simultaneously,the activation of stress related protein p38 was also restrained, while “rescuing” the activation of p38 can alleviate the inhibition of cell survival by GFAT1 knockout. Further protein mass spectrometry and immunoprecipitation analysis revealed that GFAT1 could bind to the transformation growth factor β -activated kinase 1-binding protein 1 (TAB1) in a TAB1 Ser438 phosphorylation-dependent manner, which can activate p38 in different ways. Further mechanism studies revealed that GFAT1 could facilitate the formation of the “TTLL5-GFAT1-TAB1” compound and glutamatlation modification of TAB1 Glu212, which is essential for TAB1 to recruit and activate p38 . Meanwhile, in cell physiology, the activation of GFAT1-TAB1-p38 signaling can inhibit the death and promote the proliferation of lung adenocarcinoma cells to some extent. The final clinical sample analysis suggested that the high expression of GFAT1 or the upregulation of TAB1 Ser438 phosphorylation is associated with the poor prognosis in patients with lung adenocarcinoma.
The first authors of the paper are Wei Shupei, a doctoral candidate of the SKLRD and the associate research fellow Qin Zhao (with Renji Hospital of Shanghai Jiaotong University School of Medicine); the corresponding authors of the paper are associate research fellow Chen Tao, associate professor Liang Wenhua with the SKLRD and professor Jiang Yuhui with Renji Hospital of Shanghai Jiaotong University School of Medicine. In 2015, the research cooperation on the metabolism of lung cancer cells was started by the research team led by Chen Tao and the research team led by professor Jiang Yuhui under the guidance and care of Academician Zhong Nanshan. This is another important collaboration fruit between the two sides after they jointly published their research findings “PAK4 Phosphorylates Fumarase and Blocks TGFβ -Induced Cell Growth Arrest in Lung Cancer Cells” [9] in the journal Cancer Research in 2019.
References
1. Hay, N. Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635–649 (2016).
2. Xu, D. et al. The Evolving Landscape of Noncanonical Functions of Metabolic Enzymes in Cancer and Other Pathologies. Cell Metab. 33, 33–50 (2021).
3. Akella, N. M., Ciraku, L. & Reginato, M. J. Fueling the fire: emerging role of the hexosamine biosynthetic pathway in cancer. BMC Biol. 17, 52 (2019).
4. Chiaradonna, F., Ricciardiello, F. & Palorini, R. The Nutrient-Sensing Hexosamine Biosynthetic Pathway as the Hub of Cancer Metabolic Rewiring. Cells 7, 53 (2018).
5. Li, L. et al. High expression of GFAT1 predicts unfavorable prognosis in patients with hepatocellular carcinoma. Oncotarget 8, 19205–19217 (2017).
6. Yang, C. et al. High expression of GFAT1 predicts poor prognosis in patients with pancreatic cancer. Sci. Rep. 6, 39044 (2016).
7. Moloughney, J. G. et al. mTORC2 modulates the amplitude and duration of GFAT1 Ser-243 phosphorylation to maintain flux through the hexosamine pathway during starvation. J. Biol. Chem. 293, 16464–16478 (2018).
8. Moloughney, J. G. et al. mTORC2 Responds to Glutamine Catabolite Levels to Modulate the Hexosamine Biosynthesis Enzyme GFAT1. Mol. Cell 63, 811–826 (2016).
9. Chen T. et al. PAK4 Phosphorylates Fumarase and Blocks TGFβ-Induced Cell Growth Arrest in Lung Cancer Cells. Cancer Res. 2019 Apr 1;79(7):1383-1397.
















