The team led by Professor Zhao Jincun analyses T cell response of mice infected by SARS-CoV-2 for the first time
2021-01-252085The team led by Professor Zhao Jincun of the SKLRD worked with the National Key Laboratory for Biosafety Testing (P3 Laboratory) and the National Key Laboratory for Biosafety of Pathogenic Microorganisms to transplant the human adenovirus ACE2 of novel coronavirus receptor into mice and study and analyze the characteristics and functions of T cell response in SARS-Cov-2 infected mice. The study will greatly promote the studies of immune response of the SARS-Cov-2 infected cells and provide theoretical basis and support for the analysis of the pathogenic mechanism of the SARS-CoV-2 and the research and development of vaccines for the pandemic. The research findings were published on the international authoritative journal, “Journal of Experimental Medicine (IF: 11.743) on January 19, 2021.

The receptor invaded by SARS-CoV-2 is human angiotensin-converting enzyme 2 (hACE2). The homologous receptor mouse ACE2, due to the difference in key amino acid site, cannot conduct the invasion of the virus. Therefore, we applied the Ad5-ACE2 transplant into mice model developed by our team earlier (Cell, 2020), which first identified the advantaged cell epitopes CD4+ T and CD8+ T of SARS-CoV-2 among the common inbred mice (BALB/c, C57BL/6) (as shown in Fig. 1).
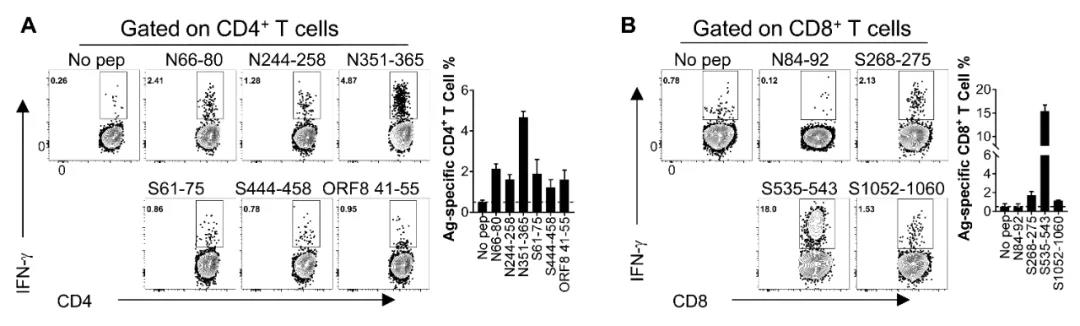
Fig. 1 The identification result of specific T cell epitope of SARS-CoV-2 among BALB/c mice
To further understand the immune response pattern and functions of specific cells triggered by the infection of SARS-CoV-2, the author analyzed the specific T cell responses in different tissues and organs of different strains of mice at different time points after getting infected by the virus and discovered that the T cell response of different infected mice reached a peak on the 8th day after the infection. The T cell response in the lungs is far higher than that in secondary lymphoid organs. The specific T cells at the infected site show stronger versatility and have a stronger killing effect towards target cells (as shown in Fig. 2). The use of the vaccine-carried immunity which only expresses T cell epitope can clear the virus more quickly after the mice are infected by SARS-CoV-2 and reduce the pulmonary pathological damage (as shown in Fig. 3).
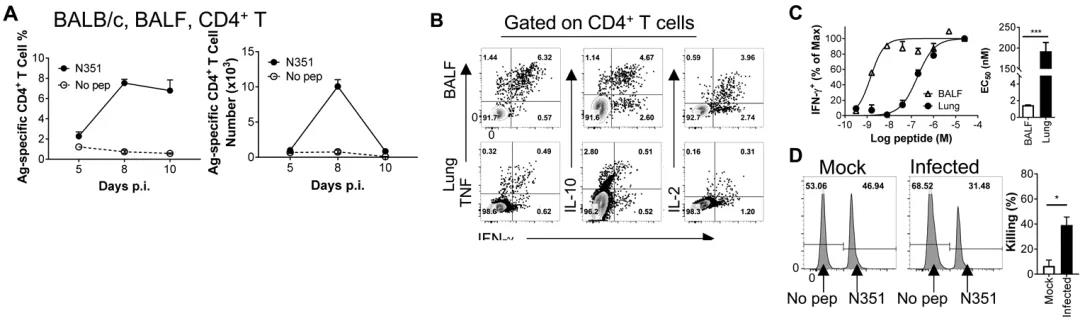
Fig. 2 The specific T cell response pattern, secretion of multiple cytokines, higher affinity and in vivo killing effect of SARS-CoV-2 among BALB/c mice
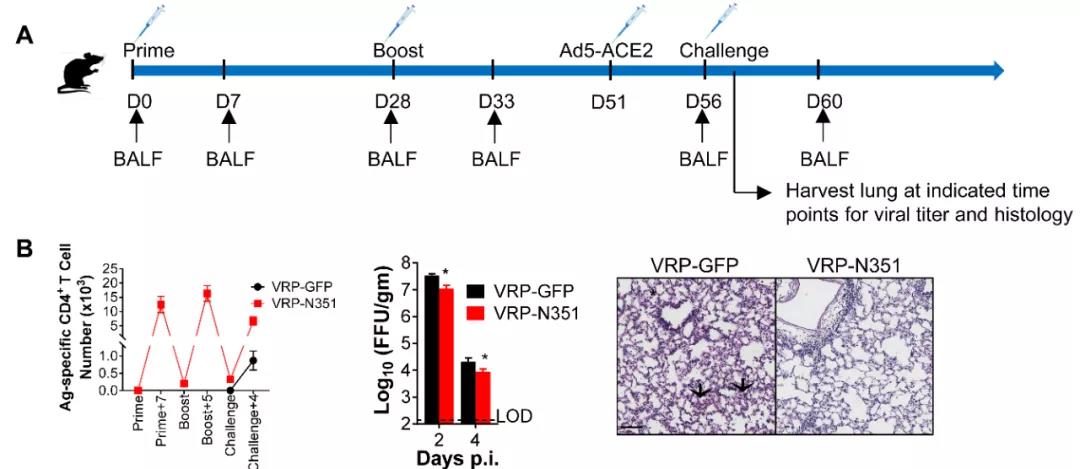
Fig. 3 The N351 specific memory CD4+ T cells can accelerate the clearing of SARS-CoV-2 from the lungs of mice and reduce the pulmonary pathological damage
In addition, this study also revealed that the I type interferon signaling pathway can “shape” the virus specific T cell response. The T cell response of mice with a deficiency in I type interferon receptor is weaker, whose ability to secrete cytokines and antigen affinity will decline. Furthermore, for the first time the author elaborated the cross-reactivity T cell response among highly pathogenic human coronavirus, which laid a foundation for the exploration and function verification of cross reaction among seven kinds of human coronavirus.
















