The SKLRD published its important research findings of treatment of SNCLC-SMAO with Bronchofiberscope injection on Lung Cancer
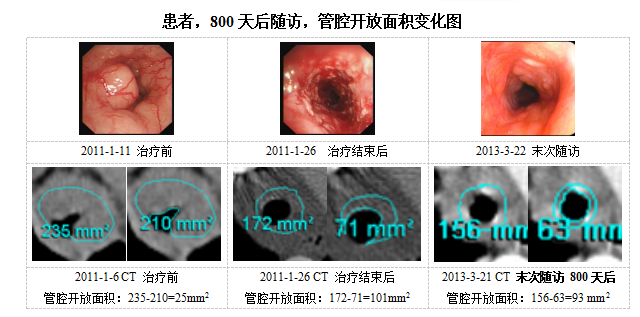
2016-06-011684Academician Zhong Nanshan, director of the State Key Laboratory of Respiratory Disease, and Professor Li Shiyue of PI took the lead to organize 17 domestic clinical research centers to carry out a multi-center clinical test on the treatment of NSCLC-SMAO with PTS. Limited by the earlier relevant requirements of the State Food and Drug Administration, the research only carries out single-arm clinical test on the most serious lung cancer (SNCLC-SMAO). In the clinical test, the researchers used Bronchofiberscope to inject a mixture of PTS and ethyl alcohol to the base of airway tumor, with a dosage of PTS/ethyl alcohol mixture at 0.1-1.5 ml in each injection and a maximum dosage of a single injection at 5.0 ml PTS. The patients need to receive 2-3 injections of Bronchofiberscope injection each week, and a course of treatment consisting of two weeks. In the first course of treatment, four PTS injections must be received by the patients at least. In the later courses of treatment, the dosage were adjusted according to the specific conditions of the patients. The primary endpoint of the research is the objective remission rate of airway obstruction evaluated under Bronchofiberscope and chest CT adopting the standards of RECIST and WHO. The secondary indicators include first second forced vital capacity, the rate of pulmonary atelectasis and survival time. The research also adopted a strict research endpoint evaluation means. In order to guarantee the evaluation accuracy under the Bronchofiberscope, the researchers used rubber ring of 1.0 cm to fix the distance from the Bronchofiberscope to the nostril in each operation. The evaluated visual field was the right middle of the airway. And a computer system was adopted to make an objective evaluation on the tumor blocking rate. In order to guarantee the accuracy of CT evaluation, an independent expert panel of imaging evaluation was invited. The data of each patient was evaluated independently by three experts.