SKLRD Reveals the Pathogenesis of Pulmonary Veno-Occlusive Disease
2020-11-031485On November 3, 2020, the pulmonary vascular disease team of the State Key Laboratory of Respiratory Disease published a basic research paper on the pathogenesis and treatment of pulmonary veno-occlusive disease, “Mitomycin C Induces Pulmonary Vascular Endothelial-to-mesenchymal Transition and Pulmonary Veno-occlusive Disease via Smad3-dependent Pathway in Rats”. Professor Wang Jian and Associate Professor Yang Kai are the co-corresponding authors of the paper, and Doctor Zhang Chenting, Professor Lu Wenju and postgraduate student Luo Xiaoyun are the co-first authors of the paper.
Pulmonary Veno-occlusive Disease is a clinicopathological syndrome involving the heart and lungs. Its clinical features are mainly progressive occlusion of pulmonary venules and capillaries, resulting in increased pulmonary vascular resistance and right-side heart failure. Its clinical manifestations are mainly occult fatigue and progressive dyspnea. In the latest clinical classification of pulmonary arterial hypertension, it is classified as a subtype of Type I pulmonary arterial hypertension. The clinical manifestations and hemadynamics of PVOD and PAH are very similar, so it is not easy to distinguish PVOD from PAH, and it is easy to misdiagnose. According to statistics, 5-25% of patients with PAH may also be patients with PVOD. The manifestations of PVOD are mainly pulmonary capillary hemangiomatosis, diffuse pulmonary venous occlusion and arterial remodeling. Unlike PAH, PVOD has no obvious fibrinoid necrosis, plexiform lesions or angiomatous lesions. It is worth noting that PAH and PVOD have completely different responses to targeted drugs for pulmonary hypertension. Patients with PVOD who use targeted drugs for pulmonary hypertension due to misdiagnosis may suffer from severe pulmonary edema. The pathogen of PVOD is not unknown yet. Therefore, an in-depth study of its pathogenesis and its difference from PAH will be instrumental in better designing personalized and efficient treatment programs for patients with PVOD.
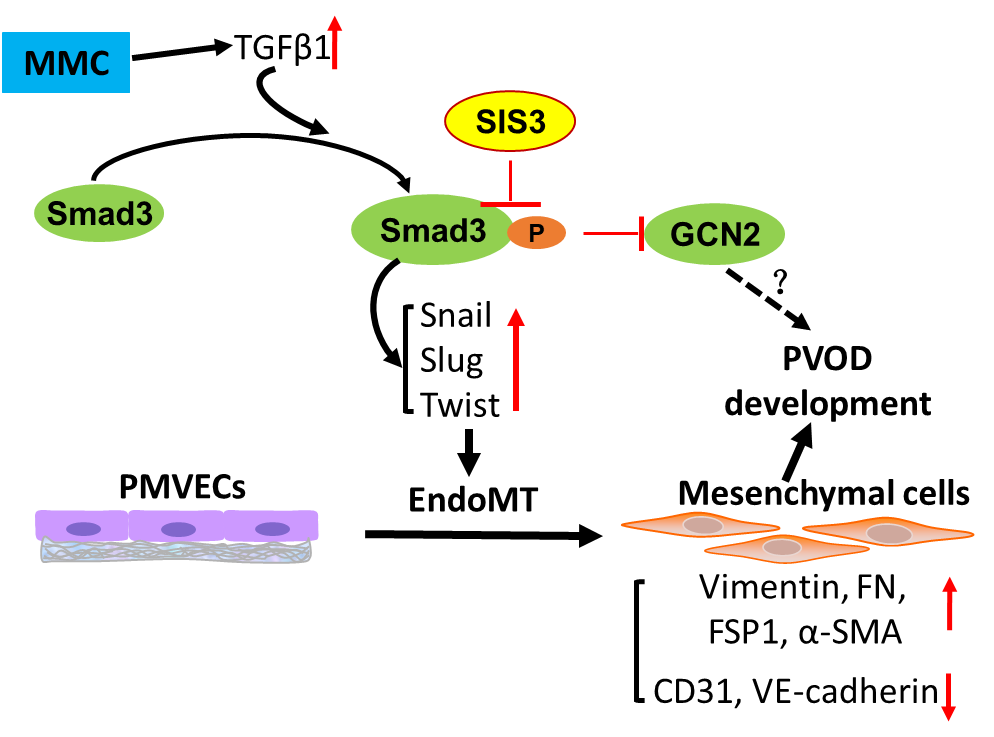
In this research, the team has successfully established a rat model of MMC-induced PVOD and found that endothelial-mesenchymal transition occurs in the lungs of patients with PVOD and rats with PVOD, causing changes in the phenotype and function of vascular endothelial cells and cellular hyperproliferation, leading to pulmonary microvascular remodeling and occlusion. Further studies have found that the TGFβ/Smad3 signaling pathway plays an important role in the process of EndoMT, while SIS3, the Smad3 inhibitor, inhibits the activation of the TGFβ/Smad3 pathway by specifically inhibiting its phosphorylation, thereby inhibiting the occurrence of EndoMT and significantly delaying the progression of PVOD in the rat model. It is expected to become a potential and effective treatment for PVOD.