The novel coronavirus IgM antibody detection kit (colloidal gold method) co-developed by SKLRD approved with Class III medical device registration certificate
2020-03-131277On March 11, the “novel coronavirus IgM antibody detection kit (colloidal gold method) was approved by the National Medical Products Administration and received a Class III medical device registration certificate (with a number 20203400199). The kit was co-developed by five organizations including the SKLRD, Guangzhou Regnerative Medicine and Health Guangdong Laboratory, Guangzhou Institute of Biomedicine and Health Chinese Academy of Science, Guangdong Hexin Health Tech Co., Ltd., and Guangzhou Enbao Biomedicine Tech Co., Ltd. The research and development of the project has won support from the Ministry of Science and Technology, Guangdong Provincial Department of Science and Technology, and the Science and Technology Bureaus of Guangzhou municipality and Guangzhou Development Zone.
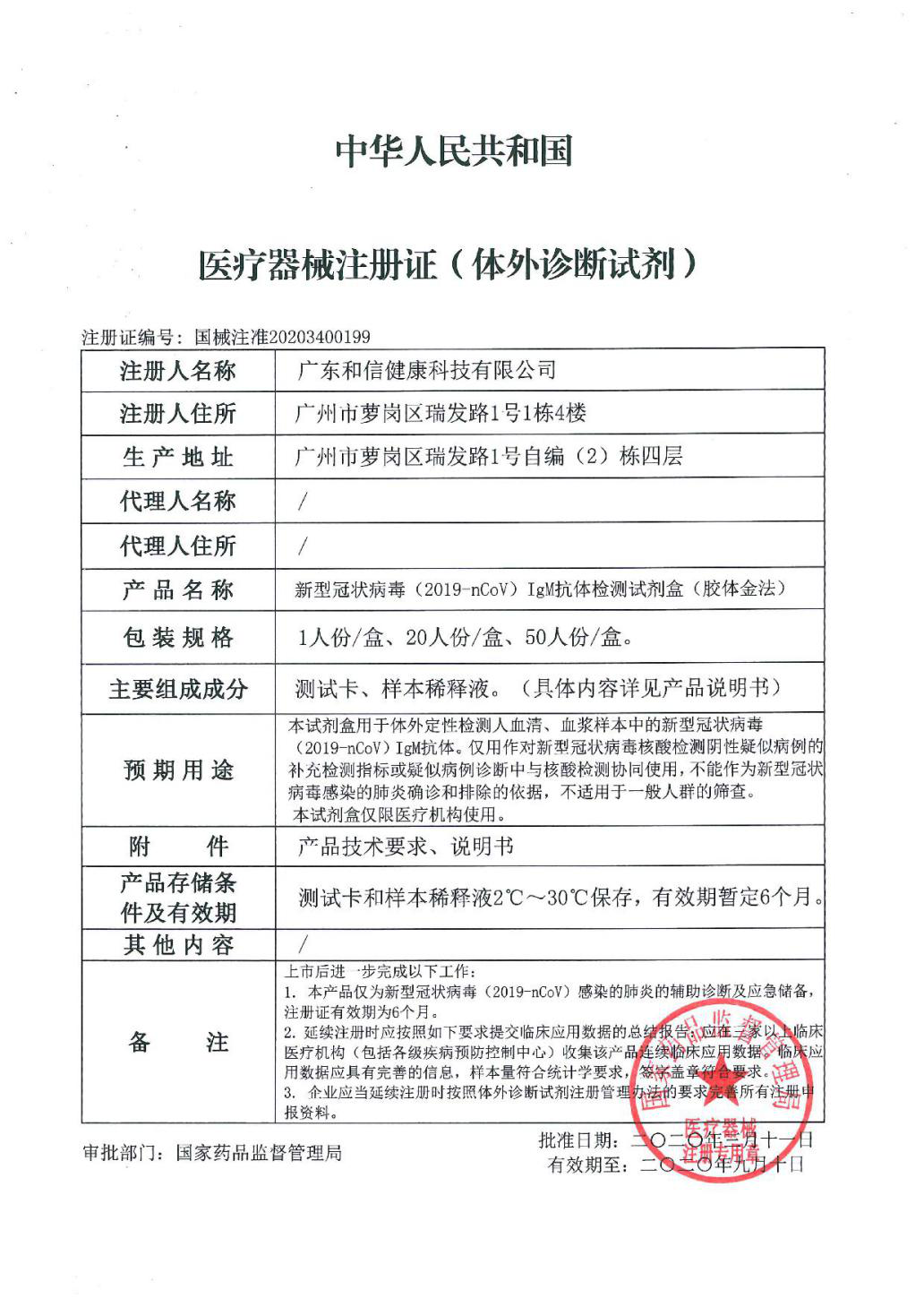
The kit is mainly used for the in vitro qualitative detection of 2019-nCoV IgM antibody in human’s serum and plasma samples. With a reaction in the ambient temperature, it can yield a result in 15 minutes, which can be used as a supplementary detection index for the suspected cases of novel coronavirus confirmed negative in the nucleic acid test or used in collaboration with nucleic acid test for the diagnosis of suspected cases.
















